HKU scientists find the H7N9 influenza virus enzootic in poultry Study published in prestigious international scientific journal, Nature
An international research team, led by Professor Yi Guan and Dr Huachen Zhu of the State Key Laboratory of Emerging Infectious Diseases and School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong (HKU), in collaboration with Shenzhen Third People?s Hospital, Shantou University Medical College and Zhejiang Provincial Center for Disease Control and Prevention, and scientists from America and Australia revealed the dissemination, divergence and establishment of the avian influenza A (H7N9) virus causing ongoing outbreaks in China. The researchers find that the H7N9 viruses have diverged into three geographically distinct clades, with at least 48 different reassortant genotypes. They also identify a previously unrecognised H7N6 avian virus in chickens. This important research will be published tomorrow (March 12) in early hours in the most prestigious international scientific journal, Nature.
About H7N9 human infections
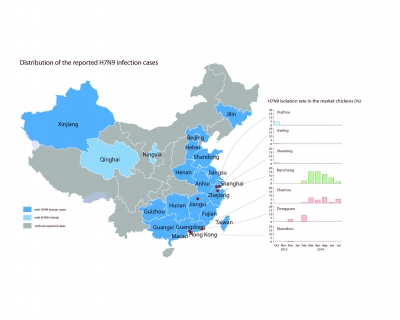
To date, a total of 638 laboratory-confirmed cases of human infection with avian influenza A (H7N9) virus have been reported globally since 2013, with 229 deaths recorded as of March 10, 2015. Human cases were not reported in Guangdong until August 2013, and the autumn of that year saw a dramatic increase of human infections. Guangdong has now become the province with the most H7N9 patients. To understand more about how the virus re-emerged, how it might develop and threaten public health, researchers from HKU and mainland Chinese institutions jointly monitored the evolution and spread of H7N9 over 15 cities across 5 provinces in China.
Key research findings
This study shows that chickens at live-poultry markets are the direct source of H7N9 human infections. The virus was predominantly shed from the respiratory and oropharyngeal tracts of these birds. At the time of the second H7N9 outbreak wave, the H7N9 isolation rate in the marketed chickens was 3% on average, yet in some cities it reached over 15%. In Jiangxi, there was a previously unrecognised, but related, H7N6 variant also emerging in chickens in the live poultry markets.
The H7N9 virus has persisted and diversified in chickens and spread across China, most likely due to poultry movement along trade routes. The expansion of its genetic diversity and geographical spread indicates that, unless effective control measures are in place, the H7N9 virus is likely to persist and spread beyond the region.
The H7N9 viruses, since their emergence in early 2013, have diverged into three geographically distinct clades that became established in East and South China. Sequential reassortments with the locally enzootic H9N2 viruses have led to the generation of at least 48 reassortant genotypes. Multiple introductions of the viruses from East China, e.g. Zhejiang, to Guangdong and the other provinces (e.g. Jiangxi) were observed. In the second wave, none of the H7N9 viruses inherited all internal genes from the Wave 1 viruses, indicating marked changes in the genotypes. Fortunately, overt antigenic changes have not been identified in the Wave 2 viruses, thus the candidate vaccine strains recommended by the WHO in 2013 will still be effective.
Research implications and suggestions
The avian H7N9 influenza viruses were predominantly isolated from market chickens which majorly replicated in the oropharyngeal and respiratory tracts of the birds, suggesting that the presence of H7N9 at live poultry markets has fuelled the recurrence of human infections. The researchers suggest that a change in the poultry trading and marketing systems to prevent direct contact of human populations with live poultry, especially chickens, will be key to averting avian-to-human transmission of viruses. Closure of live poultry markets, central husbandry and central slaughtering are the ultimate solutions to this problem.
The H7N9 influenza viruses originated in East China, disseminated across the country, and caused outbreaks in many provinces over the past two years. Effective surveillance and control measures should be reinforced on poultry especially on domestic chicken populations. Culling of birds at the infected regions and prohibition of the inter-regional poultry transportation during disease outbreaks, are the keys to break the transmission chain and to reduce the threat of H7N9 to public health.
Research method
Following the second H7N9 outbreak wave, the researchers collected samples from the oropharynx and respiratory tracts of chickens and ducks over 15 cities across 5 provinces in China. In collaboration with local hospitals and Centres for Disease Control and Prevention, the HKU researchers also collected clinical samples from patients with severe pneumonia. They isolated many influenza viruses and genetically sequenced those of the H7N9 subtype and other related viruses. Advanced phylogenetic methods were used to reconstruct how the H7N9 virus evolved, disseminated and diverged into persistent clades and to determine the origin of the genes. Of all the 709 publicly available H7N9 virus genomic sequences, 64.5% of these were contributed by this study.
About the HKU research team
This research was led by Professor Yi Guan, Daniel C K Yu Professor in Virology and Professor and Dr Huachen Zhu, Assistant Professor in the School of Public Health, Li Ka Shing Faculty of Medicine, HKU with contributions from a group of researchers from the HKU State Key Laboratory of Emerging Infectious Diseases, Shenzhen Third People?s Hospital, Shantou University Medical college, Zhejiang Provincial Center for Disease Control and Prevention, St Jude Children?s Research Hospital (USA) and Sydney University (Australia). The HKU influenza research team continues to make contributions to combat emerging infectious diseases.









.png)
