HKU study identifies host factors that function as intracellular receptors to assist Dengue Virus egress
Dengue has emerged as the most important vector-borne viral disease in tropical and subtropical areas. Membrane receptors at the surface of target cells are key host factors for virion entry; however, it is unknown whether trafficking and secretion of progeny virus require host intracellular receptors.
Researchers at the HKU-Pasteur Research Pole of the School of Public Health, Li Ka Shing Faculty of Medicine, The University of Hong Kong (HKU) have now demonstrated that host KDEL receptors (KDELRs) serve as internal receptors that interact with dengue virus (DENV) structural protein for trafficking from the Endoplasmic Reticulum (ER) to Golgi apparatus of newly formed DENV progeny virions. Besides, researchers have discovered the mechanism on preventing progeny virions to affect other healthy cells. This study, which has been published inCell Reports recently, provides new insight into the molecular mechanisms responsible for dengue progression and pathogenesis during late stage of dengue virus life cycle as well as manufacture medicine targeted to Dengue.
Research implication
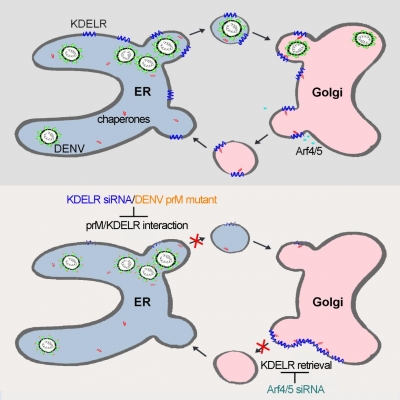
This work provides an answer to a lingering question: how does a virus that is assembled inside the cell finds its way out of it" "The virus has solved the problem by hooking to KDELRs and taking a free ride out of the ER, as these receptors travel with no passengers to the Golgi apparatus where they find their targets and bring them back to the ER. Clever and very effective as by occupying an empty receptor dengue virus does not seem to disrupt any cellular function for this part of its intracellular travel," said Dr Li Mingyuan, Research Assistant of School of Public Health, Li Ka Shing Faculty of Medicine, HKU. It has been recently suggested that KDELRs may function as signal transduction molecules. "This opens the possibility that dengue virus may usurp them to stimulate a greater flow of vesicles that are needed for its transportation along the secretory pathway," added Professor Roberto Bruzzone, visiting professor of School of Public Health, Li Ka Shing Faculty of Medicine, HKU and co-director of the HKU-Pasteur Research Pole.
Study methods and findings
The key findings of this study are that KDELRs directly interact with DENV and that depletion of KDELRs reduces secretion of dengue progeny virus from infected cells. By taking advantage of an in vitro cellular model, the authors were able to follow the intracellular journey of newly formed recombinant subviral particles (RSPs) consisting only of the virus structural envelope proteins and identify the precise step at which the journey is disrupted. Thus, RSPs remain stuck at the site of production, the ER, and are unable to move to the Golgi apparatus, the next station towards exit from the cell.
Study background
The life cycle of viruses, such as DENV, is a complex process relying on specific interactions with host factors that, in turn, represent potential targets for functionally interfering with viral replication and pathogenesis. Although the molecular identity of cellular receptors involved in virion entry has been revealed for many types of viruses, few studies have investigated whether host proteins on intracellular compartments also function as receptors to facilitate viral trafficking and release from infected cells. Thus, viral-host interactions during dengue virus egress are still poorly characterized and most cellular targets identified in high-throughput screens have not been mapped to the secretory pathway.
About the Dengue
Most people who are infected by DENV only exhibit mild influenza-like febrile symptoms and can quickly recover without complications. However, in some cases, dengue viruses cause a severe illness, that is responsible for 25,000-50,000 annual deaths each year. According to a WHO report, the incidence of dengue has increased 30-fold over the last 50 years and there are no effective drugs or vaccines available. Up to 50-100 million infections are now estimated to occur annually in over 100 endemic countries, putting almost half of the world"s population at risk.
About the DENV
DENV is a mosquito-borne virus found in tropical and sub-tropical regions of the world. Immature DENV, containing two structural glycoproteins: prM and E on its surface, is firstly assembled at the site of protein synthesis inside the cell, which is the ER. Nascent virions traverse the cell along the secretory pathway, which requires movement of virion-containing vesicular parcels from the initial assembly station, the ER, to the Golgi apparatus, with its distinct sub-compartments. Those infectious virions are eventually released from infected cells thought exocytosis and attach to another target cells which start a new infectious cycle.
About KDELRs
The three KDELRs present in the human genome are integral membrane proteins that cycle between the ER, where proteins are synthesized, and the Golgi apparatus, where proteins are sorted for secretion. The main function of KDLERs is to control the correct location of chaperones, proteins that ensure quality control of newly synthesized proteins escorting them from the ER to the Golgi apparatus. In the Golgi, chaperones dissociate from correctly folded proteins and are able to interact with KDELRs that bring them back to the ER to start a new cycle. DENV exploits the fact that KDELRs travel without passengers from the ER to the Golgi apparatus to make their way out of cells.
About the research team
The research is carried out by Dr Li Mingyuan, Research Assistant of School of Public Health, Li Ka Shing Faculty of Medicine, HKU during her PhD study. Professor Roberto Bruzzone, visiting professor of the School and Dr Wang Peigang, who is now at Key Laboratory of Protein and Peptide Pharmaceuticlals, Institute of Biophysics, Chinese Academy of Sciences, jointly supervised the research.







.png)
